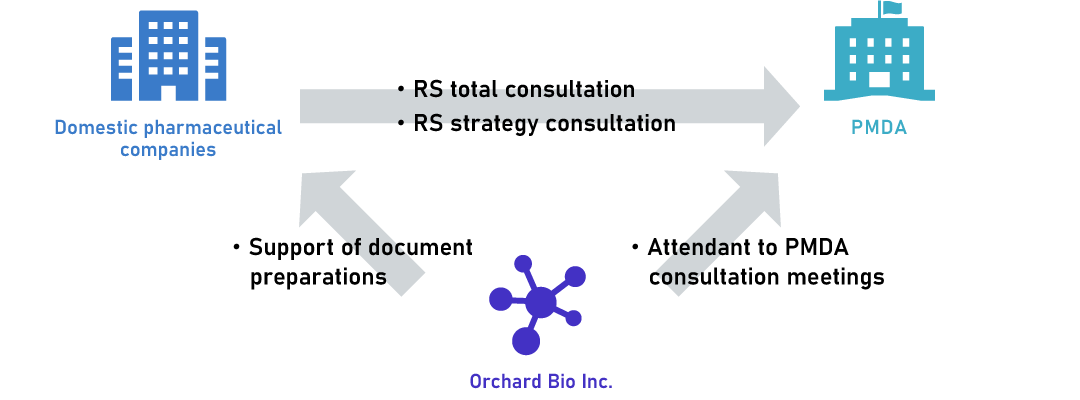
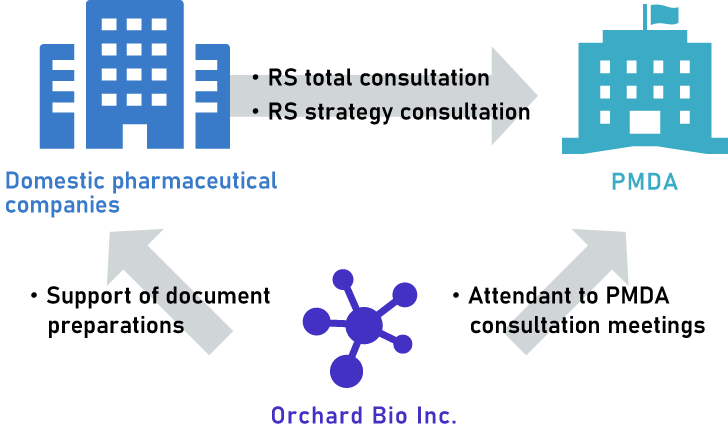
Our major CRO service to domestic companies
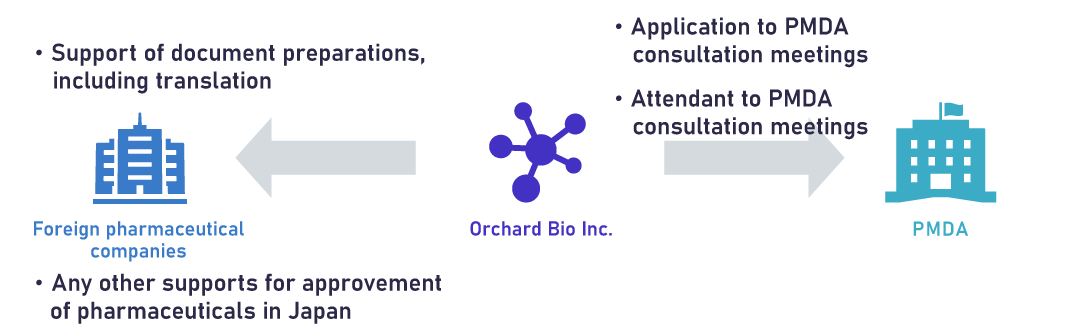
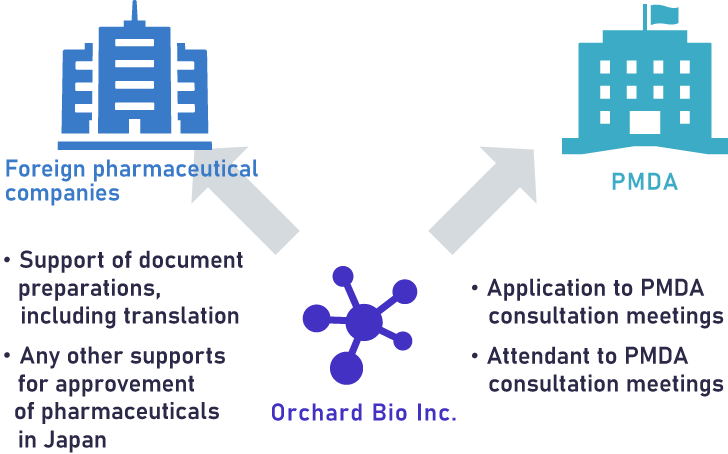
Our major CRO service to oversee companies
We also provide extensive supports to establish manufacturing process of regenerative medicines regarding to various level of facility controls and document preparations required in “ Act on Securing Safety of Regenerative Medicine (Act No. 85 of 2013)”


